Q&A
StemJournal posed some questions to Hidenori Akutsu and below you can read his answers:
Q: What is the key result of this study in relation to how this research impacts processes of ADMET related to oral drugs?
A: Drug development is an expensive endeavor. Drug companies struggle to develop drugs that are safe, effective and that can be marketed on a timely and profitable way. A large part of the investment in drug development is spent on candidate drugs that do not work due to safety or efficacy issues. In this sense, our study presents a major step to spot these issues during the pre-clinical stage. Our gut organoid enables researchers to use a system that is very similar to the human gut and that reflects very well the ADMET characteristics of drug candidates. This reduces the number of candidates to move to the next stages of development, and dramatically reduces the financial resources and the time spent in development.
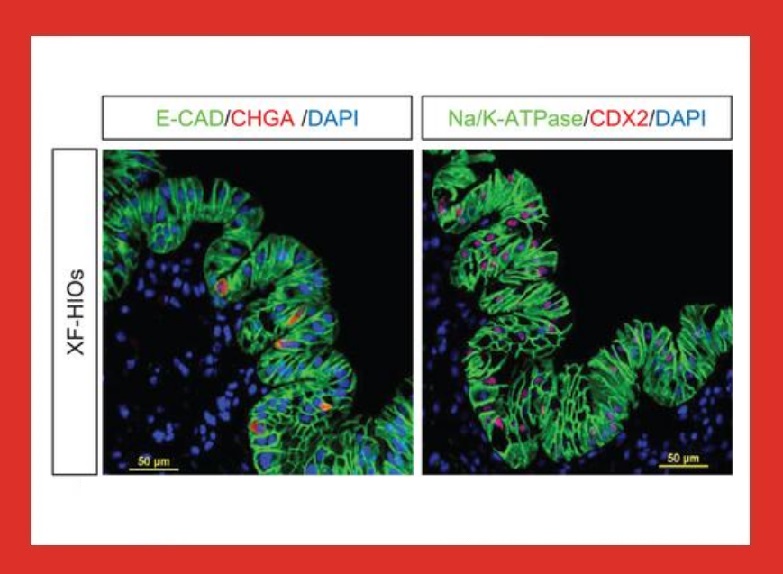
Can you explain the importance of 3D stem cell cultures for propagating human intestinal organoids?
The human body is a complex structure whose intricacies at the molecular level is only beginning to be uncovered. However, one aspect that we know since decades is that the cell communication and the intrinsic movement of organs is a three-dimensional phenomenon. Therefore, using our gut organoids to study the properties of the human gut is more appropriate than other approaches; for instance, the popular Caco-2 cells have low fidelity to human tissues both in terms of molecular composition and due to its simplistic monolayer architecture.
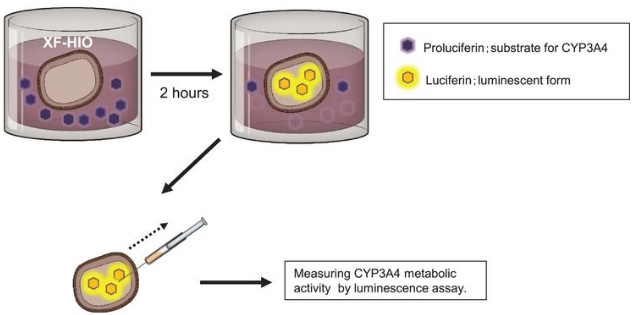
Would it be advantageous for these intestinal organoids to have transporters/metabolic properties? Is work ongoing in this regard?
The human intestine is a phenomenal structure in terms of functionality and complexity. We are studying several properties of our gut organoid to fully understand to which extent it resembles the human tissues. So far, we observed a very good correspondence between the expression of transporters in the human intestine and in our system.
What adaptations for drug screening might these organoids require in order to avoid them having limited applications?
Evidently there are aspects that are very specific for a few drugs or pharmacological questions; however, we observed a good agreement between the in vitro and the in vivo models for the most common transporters and metabolic properties.
One positive aspect of our system is its remarkable versatility; namely, we can customize it to express transporters or other factors that mimic a desired component of the real human intestine.
How can it be ascertained whether drug metabolism functions at a similar magnitude as in human intestinal tissues?
While a direct correspondence between the drug pharmacokinetics in the human intestine and in our gut organoids is not perfect, we are confident that the first line of evidence about the desirable properties of drug candidates comes from the different tissues that our organoids developed, from its peristaltic movements, and from the consistent expression of transporters related to drug metabolism. Therefore, while the organoids cannot completely answer the question of magnitude, it can still dramatically narrow down the number of drug candidates that are more likely to be safe and effective.
This is the first study to show that human gastrointestinal organoids exert catalytic activity on xenobiotics. What direction will the next phase of this research take?
There are exciting questions to pursue. Some of them are related to the intestinal microbiome. As is increasingly revealed in the scientific literature, the gut microbiome plays a major role in a plethora of physiological functions – among them, drug metabolism. Therefore, the presence of specific bacteria and certain immune cells can make our gut organoids even more realistic.
How does the content of the paper(s) relate to your ongoing research & what is your current/future focus in this field?
We believe this system is ready for application. Therefore, we want step further to collaborations with industry partners to help them succeed in their projects, and to help us understand the points that we have to improve in the next generation of gut organoids.
Anything else you’d like to share?
We are grateful to the journal for sharing our content. We look forward to an exciting future for organoid research.