Q&A
StemJournal posed some questions to Torsten and below you can read his answers:
Q: Cell replacement therapy is changing the way degenerative and chronic diseases are treated. What area do you see this impacting most? Do you have a particular interest in a certain field?
A: Due to the ease of access, congenital diseases of the bone marrow and of the eye will be the first to benefit from cell replacement therapy, particularly in combination with gene correction using one of the various genome editing tools currently available. The first clinical trials exploring stem cell-derived retinal pigmented epithelial cells (RPE) for the treatment of macular degeneration have already been completed, and gene-corrected hematopoietic stem and progenitor cells (HSPC) for beta thalassemia and sickle cell disease have entered clinical trials.
As an immunologist by training, I am very interested in engineering cells of the immune system and reprogramming them not only to fight cancer – as is being widely undertaken right now with chimeric antigen receptor (CAR) T cells – but also to treat various infectious and chronic inflammatory diseases, and to induce tolerance in autoimmunity.
Q: What are the main challenges that need to be addressed to turn universal stem cells into a viable commercial option?
A: We addressed some of the main challenges in the position paper. Most importantly, there is a demand for better animal models that predict cell engraftment in the presence of a human immune system. This should be done in close collaboration with the regulatory agencies to develop a cell product that is safe, efficacious, and of reproducible quality.
Q: Sneaking past the immune system undetected: How does that happen, and what needs improving to avoid rejection?
A: By and large, the current modifications have focused too much on T cell-mediated acute graft rejection. We know that other cell types of the immune system, such as B cells, NK cells, and other innate immune cells like macrophages and neutrophils, can contribute to allograft rejection. In order to promote durable, long-term engraftment of modified cells, chronic graft rejection in particular needs to be addressed.
Q: What have the significant advances been in genome editing to realize “off-the-shelf” cell products, and what do you see leading the way in the next 10 years?
A: We are talking about a moving target here. New advances are coming in almost weekly, and it’s difficult to predict which genome editing tool will prevail. We have witnessed the rapid transition from Zinc Fingers to TALENs to CRISPR-Cas9. More recent inventions, such as Base editing and Prime editing, developed in the lab of David Liu here at Harvard, are particularly interesting since they don’t rely on double-stranded breaks and therefore may be safer in their application to cell therapy.
Q: How do you see the “intimate collaborations” (as you call it) between academic labs, biotech and large pharma companies panning out? Is it already happening successfully?
A: The IP around our engineered immune-silent stem cells has been licensed by Harvard University to Sana Biotechnology, a necessary step for the commercial development of our technology. Early seed funding from the Harvard Stem Cell Institute (HSCI) and the Blavatnik Biomedical Accelerator, as well as the continuous support from Harvard Office of Technology Development, has proven particularly critical in the early phase of the project, where obtaining third party funding for this line of translational research was difficult.
Another example of a successful collaboration between industry and academia is Semma Therapeutics, a company based on the technology developed in Doug Melton’s lab at Harvard: the differentiation of glucose-sensitive, insulin-secreting pancreatic beta cells from iPSC for the treatment of type 1 diabetes (T1D). They have partnered and were ultimately bought by Vertex Pharmaceuticals, which will help them initiate clinical trials and – fingers crossed! – make this new form of cell therapy available to T1D patients.
Q: How does the content of the paper(s) relate to your research & what is your current/future focus in this field?
A: My personal goal is to combine cell therapy with tissue engineering. Together with the lab of Elliot Chaikof at Beth Israel Deaconess Medical Center, we are developing immune-silent universal blood vessels that one day can be used on demand for vascular reconstruction without immune rejection.
Q: Anything else you’d like to share?
A: My excitement for this highly interdisciplinary research! I would never have dreamed possible such a rapid succession of breakthroughs in genome editing that now enable the engineering of custom-designed cells, which will certainly push the boundaries of therapeutic applications.
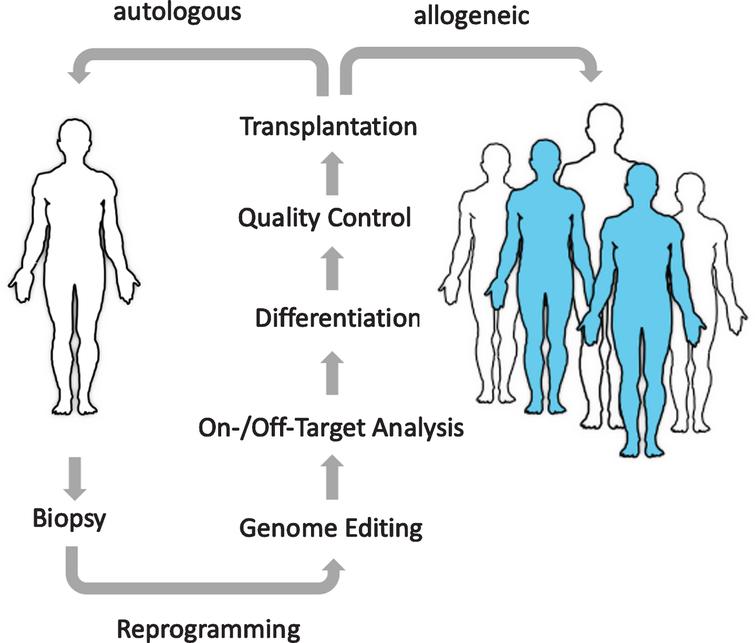
The promise of regenerative medicine: The goal of cell therapies is to replace diseased or missing cells with new healthy cells. Using universal donor cells as an unlimited, “off-the-shelf”, quality-controlled source will enable a much larger pool of patients to benefit from these emerging treatments.
--------
Thank you to Torsten for answering our questions! The StemJournal article can be viewed here.

This article is the first position paper to be published in the journal; will yours be the next? Calling all authors who have commentaries or opinion pieces that you wish to submit – or research articles, reviews or protocols for that matter – see here for full submission guidelines, or contact us if you have queries via: stemjournal@iospress.com