Saitama, Japan – A research group led by scientists from Showa University and the RIKEN Center for Biosystems Dynamics Research have, for the first time, succeeded in growing three-dimensional salivary gland tissue that, when implanted into mice, produced saliva like normal glands.
When patients lose some organ function due to disease or injury, it would be ideal to simply regrow the lost organ. But this is difficult – organogenesis is a complex and tightly regulated process that involves special stem cells that are fated to become specific tissues. However, with the exception of some organs such as hair follicles, those precursor cells are only present during early development.
The alternative is to use embryonic stem cells or induced pluripotent stem (iPS) cells – which have the ability to transform into many types of cell to create what is called an organoid – a simplified three-dimensional tissue that resembles the structure of a real organ. Growing functional organoids in the laboratory would enable patients with failing organs to recover at least some of the functions that the original organs had.
For the present study, published in Nature Communications, the researchers, led by Professor Kenji Mishima of Showa University and Takashi Tsuji of RIKEN BDR, took on the challenge of recreating salivary gland tissue. Salivary glands are important for digesting starch and for facilitating swallowing, but can be damaged by an autoimmune condition known as Sjogren’s syndrome or by radiation therapy for cancer.
These glands develop from an early structure called the oral ectoderm, but the actual process is not fully understood. It is known that organ development takes place through a complex process of chemical signaling and changes in gene expression, so the scientists began to unravel what the important changes were. They identified two transcription factors – Sox9 and Foxc1 – as being key to the differentiation of stem cells into salivary gland tissue, and also identified a pair of signaling chemicals – FGF7 and FGF10 – which induced cells expressing those transcription factors to differentiate into salivary gland tissue.
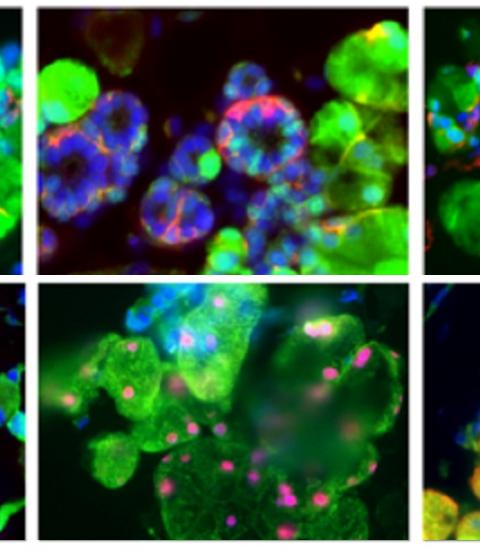
They then took on the challenge of creating an organoid. First, they used a cocktail of chemicals that allowed the formation of the oral ectoderm. They used this cocktail to induce the embryonic stem cells to form the ectoderm, and then used viral vectors to get the cells to express both Sox9 and Foxc1. Adding the two chemicals to the mix induced the cells to form tissue that genetic analysis revealed was very similar to actual developing salivary glands in the embryo.
The final step was to see if the organoid would actually function in a real animal. They implanted the organoids into actual mice without saliva glands and tested them by feeding them citric acid. When the organoids were transplanted along with mesenchymal tissue – another embryonic tissue that is important as it forms the connecting tissue that allows the glands to attach to other tissues – the implanted tissues were found to be properly connected to the nerve tissue, and in response to the stimulation secreted a substance that was remarkably similar to real saliva.
According to Kenji Mishima of Showa University, whose lab conducted the mouse experiments, “It was incredibly exciting to see that the tissues we created actually functioned in a living animal. This is an important proof of concept that organoids are a valid alternative to actual organs.”
According to Takashi Tsuji of the RIKEN Center for Biosystems Dynamics Research, who has worked on a variety of tissues such as hair and skin, “We continue to work to develop functional tissues to replace the functions of various organs, and we hope that these experiments will soon find their way into the clinic and help patients suffering from a variety of disorders.”
###
NOTES FOR EDITORS
Full Study: "Generation of orthotopically functional salivary gland from embryonic stem cells" (2018) Nature Communications, published 11 Oct 2018 (doi: 10.1038/s41467-018-06469-7). The following researchers coauthored the paper: Junichi Tanaka, Miho Ogawa, Hironori Hojo, Yusuke Kawashima, Yo Mabuchi, Kenji Hata, Shiro Nakamura, Rika Yasuhara, Koki Takamatsu, Tarou Irié, Toshiyuki Fukada, Takayoshi Sakai, Tomio Inoue, Riko Nishimura, Osamu Ohara, Ichiro Saito, Shinsuke Ohba, Takashi Tsuji, and Kenji Mishima.
Contact
Jens Wilkinson, Media Relations, RIKEN International Affairs Division (+81 48 4621225; pr@riken.jp).



