Figure 1
Click here for larger figure
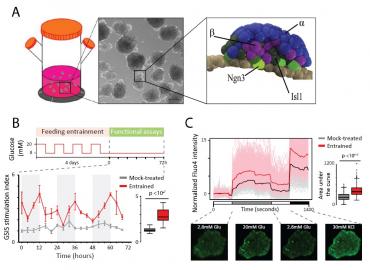
Figure 1. Circadian entrainment triggers islet organoid maturation
(A) Generation of pancreatic islet organoids. Left: organoids develop as spherical cell clusters in 3D suspension culture. Center: budding of organoids following stepwise pancreatic endocrine induction (scale bar: 200 µm). Right: model of peninsular bud structures containing a mantle of α cells (blue) and a core of β cells (purple) cells, their Ngn3+ progenitors (light green), and other Isl1+ endocrine cell types (dark green).
(B) Circadian entrainment enhances glucose-stimulated insulin secretion (GSIS). Schematic: timeline for feeding/fasting cycles conducted 4 times over 4 days, followed by GSIS assays over 72h. GSIS stimulation indexes for mock-treated vs. entrained islet organoids are shown, summarized to the right. Data are mean ±SEM of n = 3 replicate measurements.
(C) Circadian entrainment enhances glucose responsiveness. Calcium influx in mock-treated vs. entrained islets during the indicated incubations, detected using Fluo4 staining. Shown are population- and single cell-level cytosolic Ca2+ traces, summarized to the right. Traces show n = 725 cells from N = 14 islets sampled from both cultures at the 12 h circadian timepoint, each normalized to the mean of the first incubation. Bottom: representative Fluo4-staining images.
(Adapted from Sharon et al., 2019, Cell, 176, 790–804 e13 and Alvarez-Dominguez et al., 2019, Cell Stem Cell, 26, 108–122 e110.)